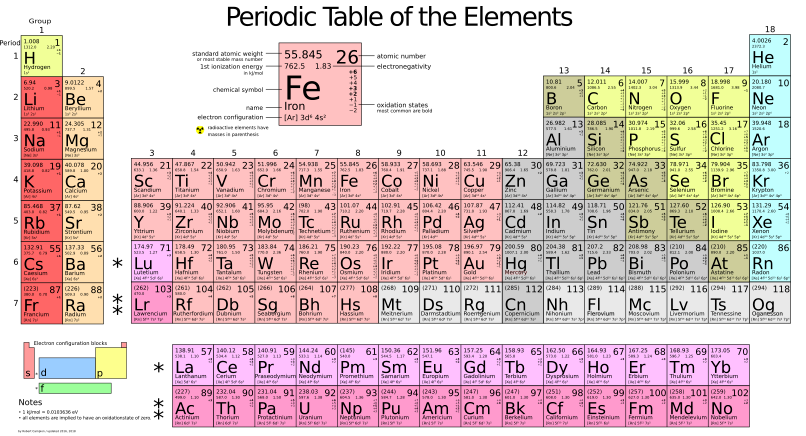
Ep. 154: The Periodic Table – Elements 21 to 30 Mnemonic
Intro
Hello and Welcome to this episode of the podcast, "The Mnemonic Tree", where we add a single mnemonic leaf to our Tree of Knowledge.
I’m Jans your Mnemonic Man and today's episode we will continue our journey down the periodic table and will be on the next ten elements from 21 to 30.
So far, we have done the first ten where the mnemonic was “Hear ye, Hear ye, Listen to BBC News On Friday Night” which stood for hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. That was episode 32.
Then we did the next ten from 11 to 20 where the mnemonic was “Story May Also Shock People, Saving Cash After Pricey Children” which stood for Sodium, Magnesium, Aluminium, Silicon, Phosphorus, Sulphur, Chlorine, Argon, Potassium, and Calcium. That was episode 131. So, if you want to brush up on the first 20 that is how to find them.
Now, into a very brief introduction. The periodic table is the arrangement of chemical elements, based on their atomic number, electron configurations, and recurring chemical properties.
These elements are organised into rows which are called periods, and columns which are called groups. There is a total of seven periods and 18 groups. The periods indicate the number of electron shells an element atom possess, while the groups indicate similar chemical properties. The table was created by Dmitri Mendeleev in 1869 and currently contains 118 elements with the addition of further synthetic elements possible in the future.
Today’s mnemonic will be on the elements in the periodic table from 21 to 30.
So, with no further ado, we will begin with a summary from Wikipedia.
Wikipedia Summary
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows ("periods") and columns ("groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics.
Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right.
The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as a dependence of chemical properties on atomic mass. As not all elements were then known, there were gaps in his periodic table, and Mendeleev successfully used the periodic law to predict some properties of some of the missing elements.
The periodic law was recognized as a fundamental discovery in the late 19th century. It was explained early in the 20th century, with the discovery of atomic numbers and associated pioneering work in quantum mechanics, both ideas serving to illuminate the internal structure of the atom.
A recognisably modern form of the table was reached in 1945 with Glenn T. Seaborg's discovery that the actinides were in fact f-block rather than d-block elements. The periodic table and law are now a central and indispensable part of modern chemistry.
The periodic table continues to evolve with the progress of science. In nature, only elements up to atomic number 94 exist;[a] to go further, it was necessary to synthesise new elements in the laboratory. By 2010, the first 118 elements were known, thereby completing the first seven rows of the table;[1] however, chemical characterization is still needed for the heaviest elements to confirm that their properties match their positions.
Some scientific discussion also continues regarding whether some elements are correctly positioned in today's table. Many alternative representations of the periodic law exist, and there is some discussion as to whether there is an optimal form of the periodic table.
Extracted from: [https://en.wikipedia.org/wiki/Periodic_table]
Mnemonic
The Periodic Table – Elements 21 to 30 Mnemonic – STaV CaMe Inside Cage of New Caledonian Zoo
(Picture your mate Stav going inside a cage with a Bengal Tiger at the New Caledonian Zoo)
21. Scandium (Sc)
22. Titanium (Ti)
23. Vanadium (V)
24. Chromium (Cr)
25. Manganese (Mn)
26. Iron (Fe)
27. Cobalt (Co)
28. Nickel (Ni)
29. Copper (Cu)
30. Zinc (Zn)
Five Fun Facts
1. Russian scientist Dmitri Mendeleyev presented his table to the Russian Chemical Society on the 6th of March 1869. His statement to the chemical society was “elements arranged according to the value of their atomic weights present a clear periodicity of properties.”
2. As of December 2018, when “The International Union of Pure Applied Chemistry” met there were 118 elements. 94 of these elements occur in nature and the rest are artificially made.
3. Metals are the most abundant in the periodic table. There are 91 metals in the periodic table, that are broken down into six groups which include:
· alkali metals
· basic metals
· alkaline earth
· lanthanides
· transition metals
· actinides
4. Many elements have the names of famous scientists as well as planets. Some of the more notable ones are Uranium named after Uranus, Mercury after Mercury, Einsteinium after Albert Einstein, Curium after Marie and Pierre Curie, and Fermium after Enrico Fermi
5. The periodic table has a cap of 137 elements! Why you ask? Well because the atoms would need to have electrons faster than the speed of light; and that by current science is not possible.
Three Question Quiz
Q.1. Which element was named after the winner of the 1921 Nobel prize for Physics?
Q.2. Is there an element in the periodic table called Neptunium? True or False
Q.3. The elements Radium and Polonium were discovered by which scientists?
Bonus Q. Which element is the basic material used in computer chips?
Bonus Q. Why should you never trust atoms?
Mnemonic Recap
The Periodic Table – Elements 21 to 30 Mnemonic – STaV CaMe Inside Cage of New Caledonian Zoo
(Picture your mate Stav going inside a cage with a Bengal Tiger at the New Caledonian Zoo)
21. Scandium (Sc)
22. Titanium (Ti)
23. Vanadium (V)
24. Chromium (Cr)
25. Manganese (Mn)
26. Iron (Fe)
27. Cobalt (Co)
28. Nickel (Ni)
29. Copper (Cu)
30. Zinc (Zn)
Three Question Quiz Answers
Q.1. Which element was named after the winner of the 1921 Nobel prize for Physics?
A. Einsteinium after Albert Einstein
Q.2. Is there an element in the periodic table called Neptunium? True or False
A. True. Named after Neptune
Q.3. The elements Radium and Polonium were discovered by which scientists?
A. Marie & Pierre Curie
Bonus Q. Which element is the basic material used in computer chips?
A. Silicon
Bonus Q. Why should you never trust atoms?
A. Because they make up everything! I’m really in my element with these chemistry puns!
Word of the Week
disingenuous
[ dis-in-jen-yoo-uhs ]
adjective
lacking in frankness, candor, or sincerity
Example
I would like to say that I could name all 118 elements in order, but that would be quite disingenuous of me. But now I can name the first 30!
Extracted from: [https://www.dictionary.com/]
👉 Free Memory Mnemonics at:
https://www.themnemonictreepodcast.com
Listen on Apple Podcasts:
https://podcasts.apple.com/au/podcast/the-mnemonic-tree-podcast/id1591795132
Listen on Spotify:
https://open.spotify.com/show/3T0LdIJ9PBQMXM3cdKd42Q?si=fqmaN2TNS8qqc7jOEVa-Cw
References
https://mytutorsource.com/blog/facts-about-periodic-table/